TransEnterix Announces LSU Health Completes Purchase of Senhance Surgical System
TransEnterix, Inc. (NYSE American:TRXC), a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced that LSU Health completed the purchase of the Company’s Senhance™ Surgical System. The Senhance has been installed at University Medical Center New Orleans (UMC New Orleans).
UMC New Orleans is home to the Reverend Avery C. Alexander Academic Research Hospital, and is an academic medical center in partnership with LSU Health New Orleans, Tulane University School of Medicine, and other local colleges and universities. UMC New Orleans will be a center for clinical excellence utilizing the Senhance Surgical System in general, colorectal and gynecologic surgery.
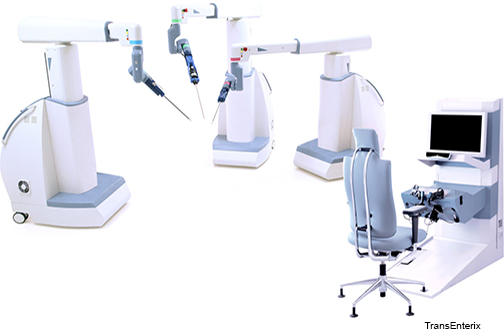
“LSU Health New Orleans and University Medical Center New Orleans see the Senhance Surgical System as an important and necessary advance in surgical care,” said Dr. Guy Orangio FACS, FASCRS, Chief Section of Colorectal Surgery at LSU Health New Orleans, and President of the American Society of Colon and Rectal Surgeons. “This new robotic system brings enabling technology with haptic feedback and innovative surgeon camera control while minimizing costs and maintaining existing efficiencies.”
“Patients are seeking minimally invasive options that utilize the most advanced technology to treat gynecologic conditions,” said Dr. Lisa M. Peacock, Chairperson of the Department of Obstetrics and Gynecology, and Section Head and Program Director of Female Pelvic Medicine and Reconstructive Surgery at LSU Health New Orleans. ”As an academic medical center, we believe this new robotic surgical platform will play an important role in our continued leadership in surgical teaching, research, and providing excellent patient care.”
On May 29, 2018, TransEnterix announced that it received FDA 510(k) clearance for laparoscopic inguinal hernia and laparoscopic cholecystectomy (gallbladder removal) procedures, doubling its total addressable market in the U.S. to over three million annual procedures. In the U.S., Senhance is now cleared for laparoscopic colorectal, gynecologic, inguinal hernia and cholecystectomy surgery.
